Misoprostol Dosing Calculator for Medical Abortion
Medical Abortion Dosing Calculator
Enter pregnancy duration to get recommended misoprostol dosing information for medical abortion (off-label use).
Recommended Regimen
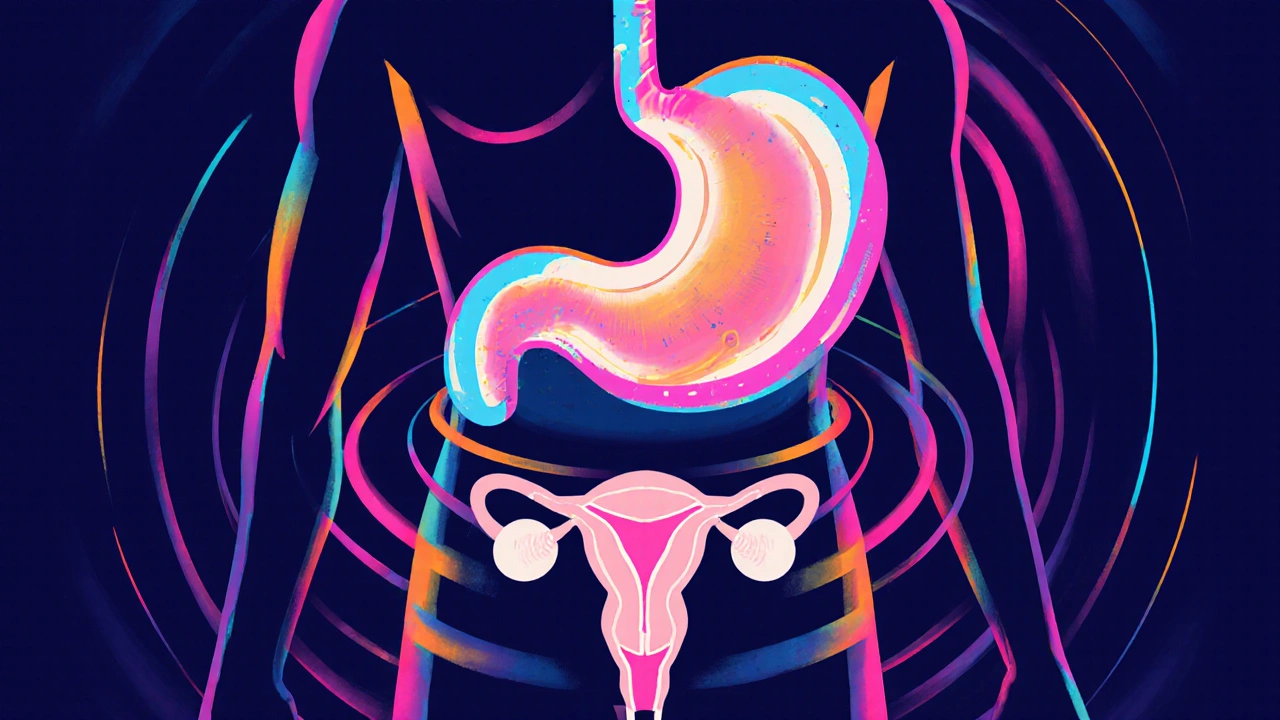
When you hear the name Misoprostol is a synthetic prostaglandin E1 analog originally approved for preventing NSAID‑induced gastric ulcers, you might think its job ends there. In practice, doctors have found dozens of ways to use it beyond the label-some life‑saving, others controversial. This guide walks through the most common off-label uses of misoprostol, explains how the dosing works, and highlights the safety checks you should expect.
How Misoprostol Works: The Pharmacology You Need to Know
Misoprostol mimics prostaglandin E1, a compound that relaxes smooth muscle, promotes cervical softening, and triggers uterine contractions. Because of these actions, it can be repurposed in any situation where you need to control the uterus or protect the stomach lining. The drug is taken orally, buccally, sublingually, or vaginally, and its effects start within 30minutes and can last up to 24hours depending on the route.
Approved (On‑Label) Indications
Regulators in the U.S., Europe, and many other regions officially allow misoprostol for two things:
- Prevention of gastric ulcers in patients taking high‑dose NSAIDs.
- Management of postpartum hemorrhage (PPH) when the uterus fails to contract after delivery.
These uses are backed by large clinical trials and appear on the label as dosage recommendations, contraindications, and side‑effect warnings.
Why Doctors Turn to Off‑Label Uses
Clinical reality often outpaces regulatory updates. When a drug shows reliable results in a new area, clinicians may adopt it while waiting for formal approval. Off‑label prescribing is legal in most countries as long as it follows evidence‑based practice and informed consent. With misoprostol, the drug’s ability to induce uterine activity opens doors to several reproductive‑health scenarios.
Off‑Label Use #1: Medical Abortion
Medical abortion is the termination of pregnancy using medication rather than surgery. A typical regimen pairs misoprostol with mifepristone, but in settings where mifepristone is unavailable, misoprostol alone can still achieve a high success rate, especially in early pregnancies (<10weeks). The common protocol involves 800µg of misoprostol taken buccally or vaginally 24-48hours after mifepristone, or 2-3 doses of 400µg every 3hours if used alone.
Success rates hover around 85‑90% for first‑trimester abortions with misoprostol alone, and side effects include cramping, bleeding, nausea, and rarely, incomplete abortion requiring surgical intervention.
Off‑Label Use #2: Cervical Ripening Before Procedures
Cervical ripening is softening and dilating the cervix to facilitate intrauterine procedures. Gynecologists often give 400µg vaginal misoprostol 12-24hours before hysteroscopy, dilation &curettage, or endometrial sampling. The drug creates a favorable cervix without the need for mechanical dilators, cutting procedure time and patient discomfort.
Evidence shows that misoprostol reduces the need for additional anesthesia and lowers the risk of cervical injury. However, patients can experience transient pain or spotting, so pre‑procedure counseling is essential.
Off‑Label Use #3: Induction of Labor
Induction of labor is the artificial initiation of uterine contractions to start childbirth. While oxytocin remains the first‑line agent, misoprostol is increasingly used when the cervix is unfavorable (Bishop score≤4) or when oxytocin alone is insufficient. Typical dosing ranges from 25µg vaginally every 4-6hours up to a maximum of 200µg, depending on hospital protocol.
Studies from 2022‑2024 report faster vaginal delivery times and lower cesarean rates with low‑dose misoprostol regimens, but the drug can cause hyperstimulation, fetal distress, or uterine rupture at higher doses. Continuous fetal monitoring is non‑negotiable.

Off‑Label Use #4: Management of Ectopic Pregnancy
Ectopic pregnancy is a fertilized egg implanting outside the uterine cavity, most often in the fallopian tube. When diagnosed early and the patient is hemodynamically stable, a combination of methotrexate and misoprostol can accelerate the resolution of the pregnancy tissue. The usual protocol involves 800µg vaginal misoprostol 24hours after methotrexate administration.
This approach shortens the time to beta‑hCG normalization and reduces the need for surgical salpingectomy. Monitoring includes serial hCG levels and ultrasound to confirm resolution.
Off‑Label Use #5: Gastric Ulcer Prophylaxis in NSAID Users (Extended Indication)
While already approved for ulcer prevention, many clinicians use misoprostol in lower‑dose regimens for patients who cannot tolerate proton‑pump inhibitors (PPIs) or have a history of PPI‑related infections. A typical dose is 200µg orally four times daily, taken with meals to maximize mucosal protection.
Side effects such as diarrhea and abdominal cramping are common, so patients are advised to stay hydrated and report severe symptoms.
Off‑Label Use #6: Post‑Abortion Hemorrhage Control
Following a medical or surgical abortion, some practitioners give 400‑600µg vaginal misoprostol to contract the uterus and minimize bleeding. The drug’s uterotonic effect helps expel retained tissue and stabilizes hemoglobin levels.
Clinical data indicate a 30% reduction in post‑abortion hemorrhage requiring transfusion when misoprostol is added to standard care.
Comparison Table: Approved vs. Off‑Label Uses
| Use | Regulatory Status | Typical Dose & Route | Clinical Setting | Major Risks |
|---|---|---|---|---|
| Gastric ulcer prophylaxis (NSAID users) | Approved | 200µg PO q6h | Outpatient, chronic NSAID therapy | Diarrhea, abdominal cramping |
| Post‑partum hemorrhage | Approved (uterine atony) | 800‑1000µg PO/IM | Delivery ward | Uterine rupture (rare), fever |
| Medical abortion (≤10weeks) | Off‑label (often combined with mifepristone) | 800µg buccal/vaginal 24h after mifepristone | Reproductive health clinic | Incomplete abortion, heavy bleeding |
| Cervical ripening before hysteroscopy | Off‑label | 400µg vaginal 12‑24h prior | Outpatient surgical suite | Cramping, spotting |
| Labor induction (unfavorable cervix) | Off‑label | 25‑50µg vaginal q4‑6h (max 200µg) | Labor & delivery unit | Uterine hyperstimulation, fetal distress |
| Ectopic pregnancy adjunct | Off‑label | 800µg vaginal 24h after methotrexate | Early‑stage ectopic, hemodynamically stable | Bleeding, tubal rupture if treatment fails |
Safety Monitoring & Contraindications
Regardless of the use case, there are common safety checkpoints:
- Pregnancy status: Misoprostol is contraindicated in women with a viable intrauterine pregnancy unless the goal is a medically supervised termination.
- Allergy to prostaglandins: Rare, but anaphylaxis can occur.
- Severe cardiovascular disease: The drug can affect blood pressure and should be avoided in unstable patients.
- Previous uterine surgery: Scar tissue raises the risk of uterine rupture during induction.
Doctors usually order baseline labs (CBC, electrolytes) and, for reproductive uses, fetal monitoring or serial beta‑hCG tests.
Legal & Ethical Considerations
Off‑label prescribing is lawful when the physician follows evidence‑based guidelines and obtains informed consent. In many countries, documentation must state the clinical rationale, dosage, and that the indication is not on the label. Failure to obtain consent can lead to malpractice claims, especially for reproductive applications.
How Doctors Decide Which Off‑Label Use to Choose
Decision‑making blends three factors:
- Evidence strength: Systematic reviews, randomized trials, or national guidelines supporting the use.
- Patient preference: Some women prefer medication over surgery; others want quickest resolution.
- Resource availability: In low‑resource settings, misoprostol may be the only option for inducing labor or managing PPH.
When all three align, misoprostol becomes a go‑to tool.
Quick Checklist for Patients Considering Off‑Label Misoprostol
- Ask why the doctor recommends an off‑label use and request the supporting evidence.
- Confirm the exact dose, route, and timing you’ll receive.
- Know the warning signs: severe abdominal pain, heavy bleeding, fever, or loss of fetal movement (if pregnant).
- Arrange for follow‑up: lab tests, ultrasound, or in‑person visits as advised.
- Carry a list of other medications you’re taking; misoprostol can interact with NSAIDs and certain antihypertensives.
Future Outlook: Will More Uses Get Official Approval?
Research pipelines are busy. The WHO’s 2025 Model List of Essential Medicines now lists misoprostol for uterine evacuation, cervical ripening, and PPH, signaling global acceptance. Ongoing phase‑III trials aim to secure FDA approval for labor induction and early‑pregnancy abortion in the United States. If those succeed, the current off‑label landscape will shift to formal labeling, simplifying prescribing and insurance coverage.
Bottom Line
Misoprostol’s versatility stems from its prostaglandin action, making it a valuable tool far beyond ulcer prevention. Whether you’re a clinician weighing risks, or a patient exploring options, understanding the dosage, setting, and safety profile is crucial. Keep the conversation open with your healthcare provider, and don’t hesitate to ask for the latest evidence that backs each off‑label application.
Frequently Asked Questions
Is it safe to use misoprostol for inducing labor at home?
No. Home induction lacks the continuous fetal monitoring and emergency support needed for uterine hyperstimulation. Hospital or birthing‑center settings are recommended.
Can misoprostol cause miscarriage in a normal pregnancy?
Yes. Misoprostol’s uterine‑contracting effect can trigger early loss if taken unintentionally during a viable pregnancy. That’s why it’s contraindicated unless the intent is a medically supervised termination.
What should I do if I experience heavy bleeding after taking misoprostol for a medical abortion?
Call your provider immediately. Heavy bleeding (>2pads/hour for more than two hours) may signal an incomplete abortion requiring surgical evacuation.
Is misoprostol covered by insurance for off‑label uses?
Coverage varies. Some insurers reimburse when the prescription is documented as medically necessary, while others consider it experimental and deny the claim. Check with your payer before starting treatment.
How does misoprostol differ from prostaglandin E2 (dinoprostone) for cervical ripening?
Both agents soften the cervix, but misoprostol is cheaper, stable at room temperature, and can be given vaginally or orally. Dinoprostone requires refrigeration and is usually more expensive. Efficacy is comparable in most studies.
Tom Green
October 17, 2025 AT 13:30Misoprostol’s versatility really shines when you look beyond the label, and clinicians have a responsibility to understand both the promise and the perils. First, the drug’s uterine contractile properties make it a valuable tool for controlling postpartum hemorrhage, a leading cause of maternal mortality worldwide. Second, its ability to soften the cervix opens doors for medical abortion protocols, especially in low‑resource settings where mifepristone isn’t available. Third, the gastric protective effect remains essential for patients on high‑dose NSAIDs, reducing ulcer incidence dramatically. Fourth, off‑label use for cervical ripening before induction of labor can shorten the time to delivery and lower the need for operative interventions. Fifth, some gastroenterologists repurpose it for managing ulcerative colitis flares, though evidence is still emerging. Sixth, the drug’s short half‑life allows for flexible dosing schedules that can be tailored to individual patient response. Seventh, careful patient selection mitigates the risk of uterine hyperstimulation, a serious concern in obstetric applications. Eighth, clinicians should always obtain informed consent, clearly explaining that the use is off‑label and reviewing potential side‑effects such as cramping, bleeding, or fever. Ninth, monitoring protocols usually include baseline vitals and follow‑up ultrasounds when used for pregnancy‑related indications. Tenth, the cost‑effectiveness of misoprostol, especially compared to dinoprostone, makes it an attractive option for health systems with limited budgets. Eleventh, pharmacy compounding must adhere to strict sterility standards to avoid contamination, particularly for vaginal administration. Twelfth, insurance coverage varies widely, so it’s prudent to verify reimbursement policies before prescribing. Thirteenth, ongoing research continues to explore novel indications, including potential roles in oncology for tumor‑associated bleeding. Fourteenth, interdisciplinary collaboration between obstetricians, gastroenterologists, and pharmacists ensures comprehensive patient care. Fifteenth, staying updated with the latest guidelines from ACOG and WHO helps clinicians use misoprostol safely and effectively. Finally, sharing real‑world experiences via case reports contributes to the collective knowledge base, ultimately improving outcomes for patients worldwide.
Emily Rankin
October 22, 2025 AT 18:30Reading about misoprostol’s off‑label journeys reminds us that medicine is as much an art as a science. The drug’s capacity to bring hope in dire situations embodies the resilience of human ingenuity. When faced with limited resources, clinicians creatively repurpose tools, turning obstacles into opportunities. It’s uplifting to see evidence‑based practice guiding these adaptations, ensuring safety while expanding access. Ultimately, each successful outcome adds a bright thread to the tapestry of patient care.
Rebecca Mitchell
October 27, 2025 AT 22:30Misoprostol works fast and lasts long enough for most protocols
Its side effects are usually manageable if patients are warned
Lauren Sproule
November 2, 2025 AT 03:30i think misoprostol is really cool its easy to store and cheap
lots of docs hav been using it for years and it helped many ppl
just make sure u follow the dosing guidelines and watch for cramps
CHIRAG AGARWAL
November 7, 2025 AT 08:30Honestly the insurance companies love to play games with misoprostol coverage. They claim it’s experimental for anything beyond ulcers, yet they refuse to pay for something that can literally save a mother’s life. This kind of greed is infuriating and needs to be called out. Doctors should push back and demand fair reimbursement, otherwise patients will suffer.
genevieve gaudet
November 12, 2025 AT 13:30From a global perspective misoprostol has been a game‑changer, especially in low‑income countrys where access to mifepristone is scarce. Its stability at room temperature means it can be shipped to remote villages without a cold chain. I’ve seen stories from field workers who call it "the lifesaver pill" because it prevents both ulcer complications and postpartum bleedings. The cultural acceptance varies, but overall it bridges gaps in reproductive health care.
Patricia Echegaray
November 17, 2025 AT 18:30Don’t be fooled by the glossy pharma brochures. Behind the scenes, big drug lobbies push misoprostol off the radar when it threatens their profit margins. They’ll hype up brand‑name alternatives, wrap them in patriotic rhetoric, and label any off‑label use as "dangerous experimentation". It’s a classic case of corporate manipulation masquerading as medical prudence.
Miriam Rahel
November 22, 2025 AT 23:30According to the latest guidelines, the recommended dosage for obstetric hemorrhage is 800‑1000 µg administered intramuscularly, repeated after 30 minutes if uterine tone remains inadequate. For cervical ripening, 25‑50 µg vaginally every 6 hours is advised, not exceeding 200 µg total. When employed for medical abortion, the regimen should consist of 800 µg sublingually followed by 400 µg vaginally after 24 hours. Rigorous adherence to these parameters minimizes adverse events and optimizes therapeutic success.
Samantha Oldrid
November 28, 2025 AT 04:30Sure, because everything’s always that simple.
lisa howard
December 3, 2025 AT 09:30The ethical nuances surrounding misoprostol’s off‑label applications are nothing short of a theatrical drama. On one hand, you have the desperate mother clutching at any lifeline to halt relentless bleeding, and on the other, the wary regulator clutching at the sanctity of the label. The tension builds as clinicians navigate the fine line between lifesaving improvisation and potential legal fallout. Every dose becomes a plot twist, each patient outcome a climax that can either vindicate or condemn the prescriber. And let’s not overlook the patients themselves, who must grapple with the emotional turbulence of making such high‑stakes decisions. In the end, the story is told not just in clinical data, but in the raw human experience that pulses through every courtroom and bedside. This saga will continue to evolve, driven by the relentless pursuit of better outcomes and the inevitable clash of policy and practice.
Cindy Thomas
December 8, 2025 AT 14:30Interesting take on the drama, but I think we’re overlooking the data that actually supports these uses. 🤔 The studies show comparable efficacy, so why let fear dictate practice? :)
Valerie Vanderghote
December 13, 2025 AT 19:30Wow, that optimism is refreshing! I’ve seen patients in rural clinics whose lives were literally changed by a simple misoprostol tablet. It’s heartbreaking when bureaucracy delays access. Hope we keep pushing for broader acceptance.
Michael Dalrymple
December 19, 2025 AT 00:30From a coaching perspective, it’s essential to empower clinicians with clear, evidence‑based guidelines. Providing education on dosing, monitoring, and informed consent builds confidence. When providers feel supported, they’re more likely to adopt off‑label strategies responsibly. This, in turn, translates to better patient outcomes across diverse settings.
Emily (Emma) Majerus
December 24, 2025 AT 05:30Totally agree – clear guidance makes all the difference. Let’s keep sharing best practices.
Virginia Dominguez Gonzales
December 29, 2025 AT 10:30There’s something profoundly moving about a pill that can both protect the stomach and rewrite the course of a pregnancy. In moments of crisis, misoprostol stands as a beacon of scientific triumph, a testament to human perseverance. When used wisely, it can transform sorrow into relief, uncertainty into hope. Let’s honor its potential by advocating for responsible, compassionate use worldwide.