When you take digoxin, you're not just swallowing a pill. You're trusting your life to a drug that has almost no room for error. Digoxin is used to treat heart failure and irregular heartbeats like atrial fibrillation. But here’s the catch: the difference between a safe dose and a dangerous one is tiny. The therapeutic range? Just 0.5 to 2.0 ng/mL in your blood. Go a little above that, and you risk vomiting, confusion, dangerous heart rhythms - even death. Go below it, and your heart condition gets worse. That’s what makes digoxin a narrow therapeutic index (NTI) drug. And when you switch from one generic version to another, things can go wrong - fast.
Why Generic Digoxin Isn’t Like Other Generics
Most generic drugs are straightforward. If they match the brand-name version in strength and how fast they dissolve, they’re approved. But digoxin? The FDA treats it differently. In 2002, the agency made a rare move: it required every generic digoxin product to prove it’s bioequivalent to Lanoxin, the original brand. That means the amount of drug your body absorbs - measured by AUC and Cmax - must fall within 80% to 125% of Lanoxin’s levels. Sounds fair, right?
But here’s the problem: bioequivalence is a group average. One study might show that 12 healthy volunteers absorb 95% of the generic compared to Lanoxin - and the FDA says it’s good to go. But what if one person in that group only absorbed 45%? That’s not unusual. And if you’re that person, your blood level could drop below the therapeutic range. You might not feel sick right away. But over time, your heart failure gets worse. Or worse - you switch to a different generic, and suddenly your levels spike because that version is absorbed more easily. That’s when toxicity hits.
What Happens When You Switch Generics
Imagine you’ve been on the same generic digoxin for two years. Your heart feels stable. Your doctor checks your blood level every few months - it’s steady at 0.8 ng/mL. Perfect. Then your pharmacy runs out of that brand. They give you a different generic. Same dose. Same pill color. Same label. But the coating, the fillers, the manufacturing process? Different. And that changes how your body absorbs it.
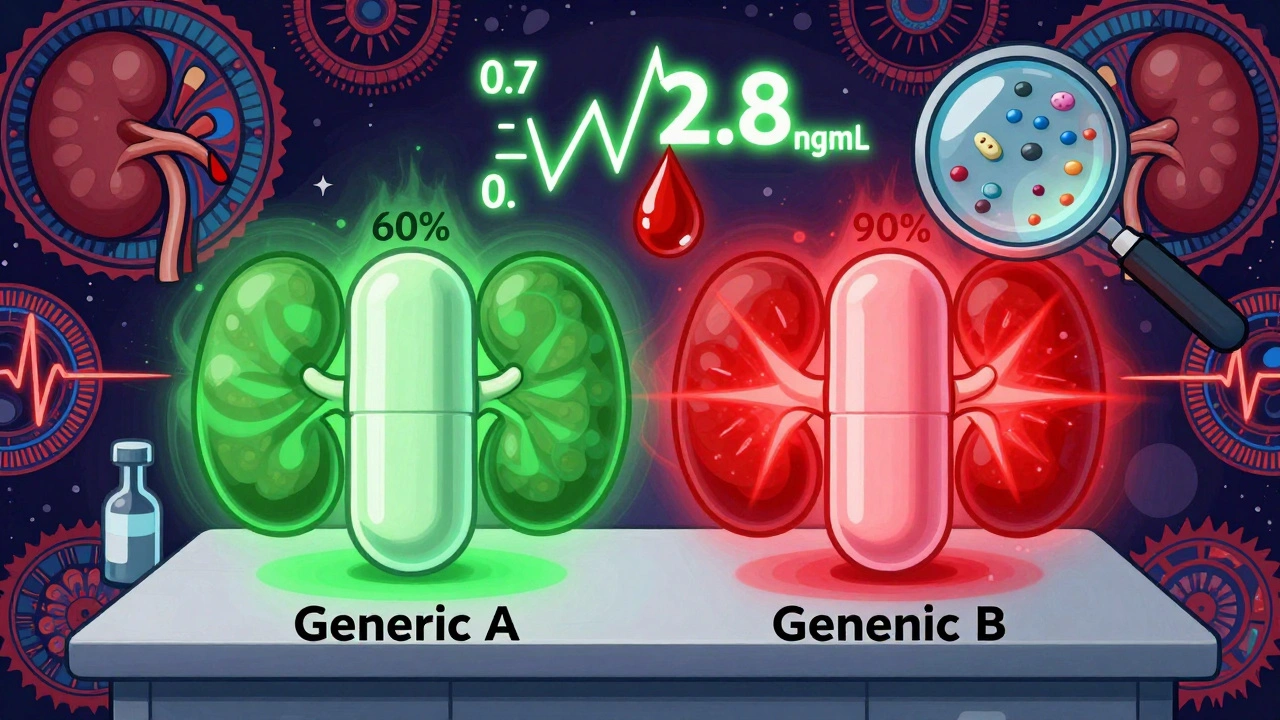
A 2022 review in the International Journal of Pharmaceutical Research found that switching between different generic digoxin products can cause blood concentration changes of more than 25%. That’s huge for a drug with such a tight window. One case report described an elderly woman who switched generics and developed severe bradycardia within days. Her digoxin level jumped from 0.7 to 2.8 ng/mL. She ended up in the ICU.
Why does this happen? Because no one tests one generic against another. The FDA only requires generics to match Lanoxin. Not each other. So Generic A might be bioequivalent to Lanoxin. Generic B might also be bioequivalent to Lanoxin. But Generic A and Generic B? They could be 30% apart in how much they deliver. And if you’re switched back and forth - maybe because of insurance changes or pharmacy stock - your body never gets a chance to stabilize.
Formulation Matters: Tablets vs. Elixir
Not all digoxin is the same, even within the same brand. The tablet form? Only about 60-80% of the dose gets absorbed. But the liquid form - the elixir - is absorbed at 70-85%. That’s a big difference. If you’re switched from a tablet to an elixir without adjusting the dose, you could overdose. Or if you’re on the elixir and your doctor switches you to a tablet without realizing the absorption difference, you might end up with subtherapeutic levels.
And it’s not just about the form. Even two tablet brands can behave differently. One might dissolve too slowly. Another might break down too fast. That’s why the FDA requires strict dissolution testing for digoxin - more than for most other generics. But even that doesn’t guarantee every batch will behave the same in every person.
Who’s at Highest Risk?
Most people taking digoxin are older adults. Many have kidney problems. That’s a double risk. Digoxin is cleared by the kidneys. If your kidneys slow down - which happens naturally with age, or because of dehydration, infection, or other meds - the drug builds up. And if you’re also switching generics? You’re playing Russian roulette with your heart.
Studies show that elderly patients are more likely to experience digoxin toxicity. Their bodies process the drug slower. They’re often on multiple other medications that interact with digoxin - like diuretics, which lower potassium and make digoxin more toxic. Or antibiotics like clarithromycin, which can raise digoxin levels by 50% or more. Add a generic switch on top of that? The risk skyrockets.
What Should You Do? Monitoring Is Non-Negotiable
There’s no way around it: if you’re on digoxin, you need regular blood tests. Not once a year. Not just when you feel bad. You need them after any change - whether it’s a new pharmacy, a different generic, a change in dose, or even a new medication.
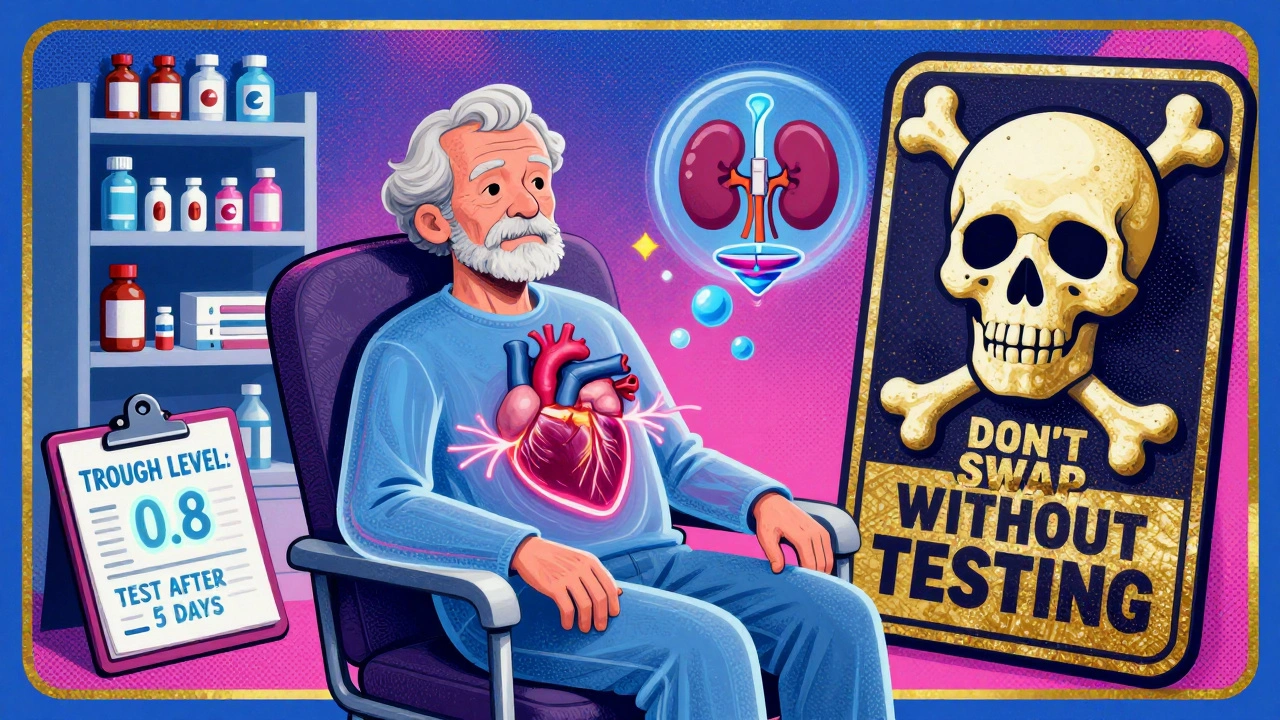
The American College of Clinical Pharmacy says to check your digoxin level just before your next dose - that’s the trough level. That’s when the drug is at its lowest, and it’s the most accurate reading. Target? 0.5-0.9 ng/mL for heart failure patients. That’s the range linked to lower death rates in recent studies. For atrial fibrillation, 0.5-2.0 ng/mL is still used, but lower is often safer.
After switching generics, wait 3 to 5 days before testing. It takes that long for the drug to reach a steady state in your blood. Don’t assume your old dose is right for the new pill. Even if your doctor says, “It’s the same dose,” it’s not the same drug in your body.
When to Call Your Doctor Immediately
You don’t need to wait for a blood test to know something’s wrong. Digoxin toxicity has clear signs:
- Nausea, vomiting, or loss of appetite
- Blurred or yellow-green vision (yes, really)
- Heartbeat that’s too slow, too fast, or irregular
- Dizziness, confusion, or extreme fatigue
If you notice any of these after switching digoxin brands - call your doctor. Don’t wait. Don’t try to “tough it out.”
What’s the Best Practice?
The American Heart Association and the American College of Cardiology both say the same thing: Stick with the same manufacturer’s product whenever possible. If your doctor prescribes a specific generic, ask for it by name. Don’t let the pharmacy swap it out without telling you. If you must switch, insist on a digoxin level check 5 days later.
Some pharmacies now offer “non-substitutable” flags for NTI drugs like digoxin. That means they can’t swap it without your doctor’s OK. Ask if yours does. If not, ask your doctor to write “Dispense as written” or “Do not substitute” on the prescription.
And if you’re on digoxin elixir - never switch to tablets without a dose adjustment. The absorption is different. Your doctor needs to know exactly what form you’re on.
The Bottom Line
Generic digoxin isn’t risky because it’s cheap. It’s risky because it’s powerful - and the body doesn’t treat all generics the same. Bioequivalence studies are designed for groups, not individuals. But your heart doesn’t care about averages. It only cares about what’s in your blood right now.
Don’t let a pharmacy switch your digoxin without telling you. Don’t assume “same dose = same effect.” And never skip your blood tests. For digoxin, monitoring isn’t optional. It’s your safety net.
If you’ve been stable on one brand for years - keep it. If you’ve had a recent switch and feel off - get tested. You’re not being paranoid. You’re being smart. Because with digoxin, the smallest change can have the biggest consequences.
Jennifer Anderson
December 6, 2025 AT 22:09just switched my digoxin last month bc my insurance dropped my brand… thought it was fine till i started feeling like death warmed over. nausea, weird vision, heart felt like it was skipping beats. went to the doc, my level was 2.9. they didn’t even tell me the pill changed. now they put me on a non-substitutable script. learn the hard way, folks.
Sadie Nastor
December 7, 2025 AT 22:58my grandma’s on digoxin and i swear she’s been fine for years… then last week she started forgetting her own name. turns out the pharmacy swapped her generic without telling anyone. she’s lucky she didn’t have a stroke. i’m now her med manager. no more swaps. ever. 🙏
Nicholas Heer
December 7, 2025 AT 23:10THIS is why the FDA is a joke. they let Big Pharma play Russian roulette with our lives. generic manufacturers are cutting corners, and the government just rubber stamps it. they don’t care if you die as long as the bill gets paid. you think this is an accident? nah. it’s profit-driven negligence. and don’t get me started on how the pharma giants own the regulators. wake up, sheeple.
Sangram Lavte
December 8, 2025 AT 08:09in India, we rarely have this problem because most digoxin is still branded. but when generics are used, doctors always check levels after switch. it’s standard. no one assumes ‘same dose = same effect’. maybe the US should adopt this habit instead of blaming pharmacies.
Oliver Damon
December 9, 2025 AT 18:30the real issue isn’t just bioequivalence-it’s the assumption that pharmacokinetics are linear across individuals. digoxin’s narrow window isn’t just about chemistry; it’s about biological variability. two people, same dose, same pill, different gut pH, different kidney function, different gut flora… and suddenly you’re in the ICU. the system treats patients as data points, not humans. we need personalized monitoring, not population averages.
Louis Llaine
December 9, 2025 AT 22:08so… you’re telling me the only reason people don’t die on digoxin is because they’re constantly getting blood drawn? cool. guess i’ll just start carrying a vial of my own blood around. 😏
Jane Quitain
December 11, 2025 AT 20:33i know someone who got switched to a new generic and ended up in the hospital… but she’s okay now! and she’s so strong for fighting through it 💪❤️ you guys are amazing for sharing this, it helps so much!!
Kyle Oksten
December 12, 2025 AT 13:18the fact that we treat digoxin like it’s ibuprofen is terrifying. this isn’t about generics being bad-it’s about regulatory laziness. if the FDA can’t enforce true interchangeability for NTI drugs, they shouldn’t approve them as interchangeable at all. simple as that.
Sam Mathew Cheriyan
December 14, 2025 AT 00:59lol you all think this is about safety? nah. it’s a psyop. the government wants us dependent on frequent blood tests so they can track us. digoxin’s just the gateway drug. next they’ll make us scan our pills with our phones. you think they don’t know what you’re taking? they’ve always known.
Ernie Blevins
December 14, 2025 AT 05:48people die on this drug every day and you’re all acting like it’s a surprise? dumbasses. just take your pills and shut up. no one cares how hard your heart is working.
Nancy Carlsen
December 14, 2025 AT 12:21my uncle was on digoxin for 12 years on the same brand. then his insurance changed and they switched him without telling him. he ended up in the hospital. now he’s fine, but he’s so grateful he found this thread. thank you for sharing this 💕 everyone deserves to know this before it’s too late. 🌸
Ted Rosenwasser
December 15, 2025 AT 23:18obviously, if you’re not monitoring your digoxin levels with HPLC-MS/MS every 72 hours, you’re not serious about your cardiac health. anyone who relies on pharmacy substitutions without a pharmacokinetic profile is essentially self-administering a time bomb. the fact that this is even a debate is embarrassing. I’ve published papers on NTI drug variability-you’d think the FDA would’ve listened by now.