When a doctor prescribes a new medication, you might assume it’s safe because the FDA approved it. But some drugs come with a hidden red flag - a black box warning. It’s not just a footnote. It’s the FDA’s loudest scream: this drug can kill you.
What Exactly Is a Black Box Warning?
A black box warning is the most serious safety alert the U.S. Food and Drug Administration can require on a prescription drug. It’s printed in a thick black border at the very top of the drug’s official prescribing information - the document doctors and pharmacists use to understand how to use a medicine safely.You won’t see it on the pill bottle. You won’t see it in TV ads. But if you dig into the full prescribing details - whether you’re a patient reading them or a doctor reviewing them - you’ll find it. It’s there to make sure no one misses it.
The FDA introduced this system after the Kefauver-Harris Amendments of 1962, when drug safety became a national priority. But the black box format itself wasn’t standardized until later. Today, it’s the gold standard for communicating life-threatening risks.
Over 400 prescription medications in the U.S. currently carry this warning, according to 2022 data. That includes drugs for diabetes, depression, pain, and even some cancer treatments. It doesn’t mean they’re all dangerous. It means the risks are serious enough that a doctor must pause, think, and talk to you before prescribing.
Why Does the FDA Use Black Box Warnings?
The FDA doesn’t slap these on lightly. They only require a black box warning when evidence shows a drug can cause death or serious injury - and that risk is preventable if handled correctly.There are three main reasons a drug gets this label:
- The side effect is so dangerous that it could outweigh the benefits for some patients.
- The risk can be reduced with careful monitoring - like regular blood tests or avoiding other drugs.
- The drug should only be used by certain people - like adults, not teens, or patients without specific health conditions.
For example, some antidepressants carry black box warnings for increased suicide risk in young adults under 25. Opioid painkillers warn of life-threatening breathing problems. Some diabetes drugs carry warnings about heart failure or pancreatitis.
The goal isn’t to scare people off. It’s to make sure the right people get the right treatment - and that everyone understands the stakes.
How Is It Different From Other FDA Warnings?
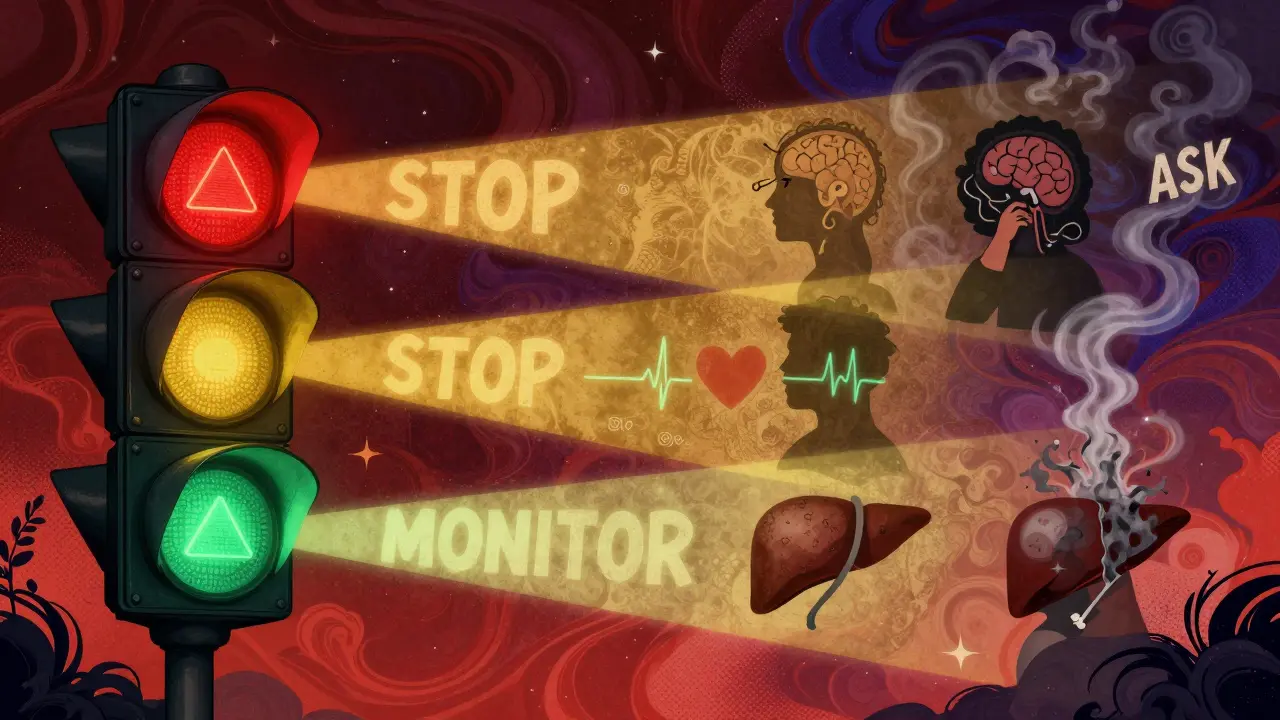
The FDA issues many kinds of safety alerts. There are general warnings, precautions, contraindications, and drug safety communications. But none are as serious as the black box.Think of it like a traffic light:
- Regular warnings? Yellow light - proceed with caution.
- Black box warning? Red light - stop and reassess.
A black box warning isn’t just a note in the middle of a long document. It’s front and center. It’s the only FDA requirement that forces the warning to appear in promotional materials, package inserts, and even digital prescribing systems.
And the impact? Real. When the FDA issued a black box warning for the diabetes drug rosiglitazone in 2007, prescriptions dropped by 70%. That’s millions of patients changing treatment. But here’s the catch: another similar drug, pioglitazone, got a similar warning - but prescriptions didn’t drop nearly as much. Why? Because rosiglitazone got far more media attention. The warning alone isn’t enough. Awareness matters.
What Should You Do If Your Medication Has a Black Box Warning?
If your doctor prescribes a drug with a black box warning, don’t panic. But do ask questions.Here’s what you need to know:
- What’s the specific risk? (e.g., liver damage, heart attack, suicidal thoughts)
- How likely is it to happen? (The warning won’t tell you this - ask your doctor for numbers)
- Are there safer alternatives?
- Do I need blood tests or regular check-ups?
- What symptoms should I watch for and call you about?
According to the American Academy of Family Physicians, doctors should use the STEPS approach when considering these drugs: Safety, Tolerability, Effectiveness, Price, Simplicity. You can use the same framework.
And if you’re unsure? Get a second opinion. Talk to a pharmacist. Use trusted resources like the Drug Effectiveness Review Project or Consumer Reports’ Best Buy Drugs list to compare options.
How Do Doctors Decide to Prescribe These Drugs?
Doctors don’t avoid black box drugs out of fear. They use them when the benefits clearly outweigh the risks.Take a patient with treatment-resistant depression. Standard antidepressants didn’t work. One option left? A drug with a black box warning for suicidal thoughts. But for this person, the alternative is no relief at all. So the doctor prescribes it - with strict monitoring, weekly check-ins, and a safety plan.
Pharmacists like Dr. Meghan Lehmann from Cleveland Clinic say it plainly: A black box warning doesn’t mean don’t take it. It means understand it.
Doctors are required to inform patients about the risks. But they’re also trained to weigh individual factors: age, other health conditions, other medications, lifestyle. A warning that’s too risky for one person might be life-saving for another.
What Happens After the Drug Is on the Market?
Most black box warnings aren’t added during clinical trials. They come later - after thousands or even millions of people have taken the drug.The FDA monitors safety through the FDA Adverse Event Reporting System (FAERS). Anyone - patients, doctors, pharmacists, even drug companies - can report side effects. That’s how the FDA finds hidden dangers.
For example, a rare but deadly liver reaction might only show up after 10,000 patients use a drug. That’s when the FDA reviews the data, talks to experts, and decides: Is this serious enough for a black box?
That’s why your drug might get a black box warning years after you’ve been taking it. It’s not a mistake. It’s how the system works.
Can These Warnings Be Removed?
Yes. If new evidence shows the risk is lower than thought - or if better monitoring makes the danger manageable - the FDA can remove or modify the warning.It’s rare, but it happens. The FDA updates warnings constantly. In 2023, several psychiatric and diabetes drugs had their labeling revised based on new long-term studies.
And sometimes, patient advocacy groups push for changes. If enough people report serious side effects, the FDA has to respond.
What’s Next for Black Box Warnings?
Experts agree: the system works - but it’s outdated.Right now, warnings say things like “may cause serious liver injury.” But they rarely say how often. Is it 1 in 10,000? 1 in 100? That’s the difference between rare and common.
The FDA has signaled it wants to improve this. Future warnings may include clearer risk numbers, plain language, and even visual cues. The goal? Help patients and doctors make smarter choices - not just react to fear.
Until then, the black box remains the strongest tool we have. It’s not perfect. But it saves lives.
How to Stay Safe
Here’s your quick action plan:- Always read the full prescribing information - ask your pharmacist for a copy.
- Ask your doctor: “Does this drug have a black box warning? What does it mean for me?”
- Know the signs of the specific risk listed - and when to call for help.
- Report any strange side effects to the FDA through MedWatch - it helps others.
- Don’t stop taking the drug without talking to your doctor. Some risks are worse than stopping cold turkey.
Medicines are powerful. So are warnings. The black box isn’t a death sentence. It’s a conversation starter. And you deserve to have that conversation.
Do all drugs with black box warnings cause serious harm?
No. A black box warning means the drug has the potential to cause serious harm - but only in certain people or under certain conditions. Many patients take these medications safely for years. The warning exists so doctors and patients can make informed decisions, not avoid the drug entirely.
Can I refuse a drug with a black box warning?
Absolutely. You have the right to ask for alternatives, refuse a prescription, or request more information. Your doctor should respect your decision. If they pressure you, it’s okay to seek a second opinion.
Why don’t I see black box warnings on over-the-counter drugs?
Black box warnings only apply to prescription drugs. The FDA regulates OTC medications differently. They’re designed to be safer for self-use, with lower risk profiles. If an OTC drug has serious risks, it’s usually pulled from the market entirely - not just labeled.
Can a black box warning be added after I’ve been taking a drug for years?
Yes. Most black box warnings are added after a drug has been on the market for years. The FDA uses real-world data from millions of patients to spot rare or delayed side effects. If new evidence emerges, they update the warning - even if you’ve been taking the drug safely.
How do I report a side effect from a drug with a black box warning?
Use the FDA’s MedWatch program. You can report online at fda.gov/medwatch, by phone, or through your doctor. Your report helps the FDA track patterns and decide whether to strengthen warnings, update labels, or even remove a drug from the market.
What to Do If You’re Concerned
If you’re taking a drug with a black box warning and feel uneasy, schedule a visit with your doctor or pharmacist. Bring the prescribing leaflet. Write down your questions. Don’t rely on Google or social media - talk to someone trained in medication safety.The system isn’t perfect. But when used right - with open communication, careful monitoring, and informed consent - black box warnings help prevent tragedy. They’re not meant to scare you. They’re meant to keep you safe.

Henry Sy
January 14, 2026 AT 11:50So let me get this straight - the FDA puts a giant black box on drugs that might kill you, but you still gotta trust the same people who let OxyContin out like it was candy? Yeah right. I’ve seen people on these meds turn into zombies, then get blamed for ‘not following instructions.’ Like the drug companies didn’t fund the studies that got this approved in the first place.
shiv singh
January 15, 2026 AT 18:54They don’t want you to know this but black box warnings are just a PR move. The real problem? Pharma owns the FDA. They don’t remove drugs - they just slap on a warning and keep selling. I know a guy who lost his liver over one of these. No one apologized. No one went to jail. Just another ‘informed consent’ checkbox.
Vicky Zhang
January 17, 2026 AT 12:03I had to take a black box drug after my depression hit rock bottom. I was terrified. But my doctor sat with me for an hour, explained every risk, and we made a plan. I’m still here. Two years later. I’m not ‘cured’ - but I’m alive. And I’m not ashamed. This isn’t about fear. It’s about having someone who cares enough to walk you through the fire.
Jason Yan
January 19, 2026 AT 07:41You know what’s wild? We treat drugs like they’re magic bullets - take one pill, fix your brain, fix your pain, fix your life. But the black box warning? It’s the system whispering: ‘This isn’t magic. This is biology. And biology is messy.’ We want quick fixes, but real healing? It’s slow. It’s scary. It requires listening. And we’ve forgotten how to listen - to doctors, to our bodies, to the warnings.
Andrew Freeman
January 20, 2026 AT 13:34lol why do we even have these warnings if no one reads em? I saw a guy on tiktok say his mom died from a black box drug and he blamed the doctor. Bro she was on 7 other meds and skipped her blood tests for 6 months. The warning was right there in the pamphlet. You just gotta read.
Sarah -Jane Vincent
January 21, 2026 AT 18:44Oh please. Black box warnings are just corporate theater. The FDA’s been corrupted since the 90s. You think they’d let a drug that kills 1 in 500 people stay on the market if they weren’t paid off? And don’t get me started on how they ignore reports from overseas. The EU banned three of these drugs years ago. But here? We’re still pushing them like vitamins. Wake up people.
Sarah Triphahn
January 23, 2026 AT 03:35People who take black box drugs are just gambling with their lives. You think you’re special? You’re not. The warning exists because people like you think ‘it won’t happen to me.’ Spoiler: it does. And then you blame the system. You’re not a victim. You’re negligent.
Susie Deer
January 23, 2026 AT 21:59Why are we letting foreign pharma companies dictate our medicine? This is why America needs to make its own drugs. No more FDA appeasing big pharma from Switzerland. We need American-made meds with American warnings. And no more black boxes - just ban the bad ones and move on.
Allison Deming
January 24, 2026 AT 21:20While I appreciate the article’s thoroughness, I must emphasize that the ethical imperative to disclose material risks is not merely regulatory - it is a foundational tenet of medical autonomy. The black box warning, as a mechanism of informed consent, operates within a legal and moral framework that transcends commercial interests. To dismiss it as performative is to misunderstand the very architecture of patient rights.
Robert Way
January 25, 2026 AT 06:48my doc gave me this drug with a black box and i didnt even know till i saw the bottle. he just said ‘it works’ and handed me the script. now i got liver issues. i dont even know if its the drug or my bad diet. he never said anything about tests. why didnt he tell me? i trusted him.
says haze
January 26, 2026 AT 19:58It’s fascinating how society reduces complex pharmacological risk to a single visual cue - a black box - as if that somehow encapsulates the entire ontological weight of human vulnerability. We’ve outsourced moral responsibility to typography. The real tragedy isn’t the drug. It’s that we’ve stopped thinking. We’ve turned medicine into a menu. And now we’re surprised when the entrée comes with side effects.
Dylan Livingston
January 28, 2026 AT 11:32Oh honey. You really think the FDA cares about you? They’re just waiting for you to die so they can add another black box and pat themselves on the back. Meanwhile, the drug maker made $2 billion last year. You’re not a patient. You’re a data point. And your suffering? That’s just the cost of doing business in the land of the free and the home of the pill.
Anna Hunger
January 29, 2026 AT 22:00It is imperative to underscore that the presence of a black box warning does not constitute a contraindication, but rather a clinical flag requiring individualized risk-benefit analysis. Patients who are well-informed, monitored appropriately, and engaged in shared decision-making often achieve favorable outcomes despite these warnings. The onus is not on the medication - it is on the healthcare ecosystem to communicate with precision, diligence, and compassion.
TooAfraid ToSay
January 29, 2026 AT 22:39They say black box warnings save lives. But I’ve seen families torn apart because someone got scared and quit their meds cold turkey. Now they’re in the ER with withdrawal psychosis. The warning didn’t save them. The panic did. The system doesn’t warn you about the fear. It just gives you a box. And then leaves you alone with it.